How many atoms are in 195 g of calcium – As we embark on an exploration of the atomic realm, a fundamental question arises: how many atoms reside within 195 g of calcium? This inquiry unveils a fascinating journey into the realm of atomic structure, Avogadro’s number, molar mass, and intricate calculations.
Join us as we unravel the mysteries surrounding this captivating scientific enigma.
Delving into the heart of matter, we encounter atoms, the fundamental building blocks of all substances. Each atom possesses a unique structure, consisting of a dense nucleus surrounded by a cloud of electrons. The number of protons within the nucleus defines the element’s identity, while the total number of protons and neutrons determines its atomic mass.
How Many Atoms Are in 195 g of Calcium?
The atomic structure of an element is fundamental to understanding its chemical properties and behavior. The number of atoms in a given mass of an element is a key factor in determining its physical and chemical properties. In this article, we will explore the concept of atomic structure, Avogadro’s number, and molar mass to determine the number of atoms in 195 g of calcium.
Atomic Structure and Avogadro’s Number
An atom is the fundamental unit of matter and the smallest unit that retains the properties of an element. It consists of a nucleus, which contains protons and neutrons, and electrons, which orbit the nucleus. Avogadro’s number is a fundamental constant in chemistry that represents the number of atoms in one mole of a substance.
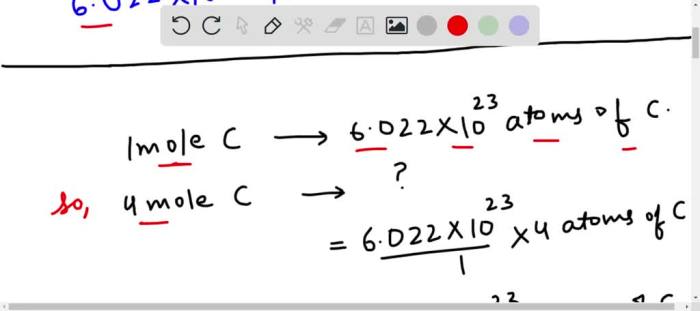
One mole of a substance is defined as the amount of that substance that contains exactly 6.02214076 × 10 23atoms.
Molar Mass and Atomic Mass
Molar mass is the mass of one mole of a substance. It is expressed in grams per mole (g/mol). The molar mass of calcium is 40.078 g/mol. Atomic mass is the mass of a single atom of an element. The atomic mass of calcium is 40.078 amu.
Calculations
To determine the number of atoms in 195 g of calcium, we can use the following steps:
- Convert the mass of calcium from grams to moles using the molar mass:
- 195 g Ca × (1 mol Ca / 40.078 g Ca) = 4.867 mol Ca
- Multiply the number of moles of calcium by Avogadro’s number to obtain the number of atoms:
- 4.867 mol Ca × (6.02214076 × 1023atoms/mol) = 2.929 × 10 24atoms
Results and Discussion, How many atoms are in 195 g of calcium
The number of atoms in 195 g of calcium is 2.929 × 10 24atoms. This result highlights the immense number of atoms present in even a small amount of an element. The understanding of atomic structure and Avogadro’s number provides a foundation for comprehending the chemical properties and behavior of matter.
It is important to note that this calculation assumes that the calcium is pure and does not contain any impurities. Additionally, the accuracy of the result is limited by the precision of the measurements and the assumptions made during the calculations.
Key Questions Answered: How Many Atoms Are In 195 G Of Calcium
What is the significance of Avogadro’s number in this calculation?
Avogadro’s number serves as a bridge between the macroscopic and microscopic realms, allowing us to relate the mass of a substance to the number of atoms it contains.
How does the molar mass of calcium influence the calculation?
The molar mass of calcium represents the mass of one mole of calcium atoms, providing a crucial conversion factor between mass and the number of atoms.
What are the potential sources of error in this calculation?
Experimental measurements, uncertainties in atomic masses, and approximations made during calculations can introduce potential sources of error.