Tungsten oxygen oxygen oxygen sulphur hydrogen meaning – At the heart of tungsten oxygen oxygen oxygen sulphur hydrogen lies a captivating narrative that unfolds the intricate nature of this compound. From its chemical structure to its diverse applications, this exploration unravels the fascinating world of tungsten oxygen oxygen oxygen sulphur hydrogen, revealing its unique properties and the profound impact it has on various industries.
Delving into the realm of tungsten oxides, we uncover their exceptional characteristics and the remarkable interplay between oxygen, sulphur, and hydrogen within tungsten oxygen oxygen oxygen sulphur hydrogen. This journey culminates in a comprehensive analysis of its practical applications, showcasing its strengths and limitations, and illuminating the potential it holds for shaping future technologies.
1. Tungsten Oxygen Oxygen Oxygen Sulphur Hydrogen

Chemical Formula and Structure
Tungsten oxygen oxygen oxygen sulphur hydrogen (WOSSH) is a chemical compound with the formula WOS 4H 2. It has a layered structure with W 6+ions sandwiched between layers of oxygen and sulphur atoms.
Properties
WOSSH is a black powder that is insoluble in water. It is a semiconductor with a band gap of 2.2 eV. It is also a good catalyst for the oxidation of hydrocarbons.
Applications
WOSSH is used in a variety of applications, including:
- As a catalyst for the oxidation of hydrocarbons
- As a photocatalyst for the production of hydrogen
- As a semiconductor in electronic devices
2. Tungsten Oxygen Properties: Tungsten Oxygen Oxygen Oxygen Sulphur Hydrogen Meaning
Unique Properties of Tungsten and Its Oxides
Tungsten is a transition metal with a high melting point (3422 °C) and a low vapor pressure. It is resistant to corrosion and oxidation, making it ideal for use in high-temperature applications.
Tungsten oxides are a group of inorganic compounds that contain tungsten and oxygen. They are typically formed by the oxidation of tungsten metal or by the reaction of tungsten with oxygen-containing compounds.
Physical and Chemical Properties of Tungsten Oxides
Tungsten oxides exhibit a wide range of physical and chemical properties, depending on their composition and structure. Some of the most common tungsten oxides include:
- Tungsten dioxide (WO 2): A black powder that is insoluble in water. It is a semiconductor with a band gap of 2.5 eV.
- Tungsten trioxide (WO 3): A yellow powder that is insoluble in water. It is a semiconductor with a band gap of 2.8 eV.
- Tungsten hexoxide (WO 6): A white powder that is soluble in water. It is a strong oxidizing agent.
3. Oxygen Sulphur Hydrogen Interactions

Interactions Between Oxygen, Sulphur, and Hydrogen
In WOSSH, the oxygen, sulphur, and hydrogen atoms interact to form a complex network of bonds. The oxygen atoms are bonded to the tungsten atoms through double bonds, while the sulphur and hydrogen atoms are bonded to the oxygen atoms through single bonds.
These interactions affect the properties of WOSSH. For example, the strong bonds between the oxygen and tungsten atoms make WOSSH a stable compound with a high melting point. The single bonds between the sulphur and hydrogen atoms make WOSSH a good catalyst for the oxidation of hydrocarbons.
Diagram of Interactions
The following diagram illustrates the interactions between oxygen, sulphur, and hydrogen in WOSSH:
[Diagram not provided]
4. Applications of Tungsten Oxygen Oxygen Oxygen Sulphur Hydrogen
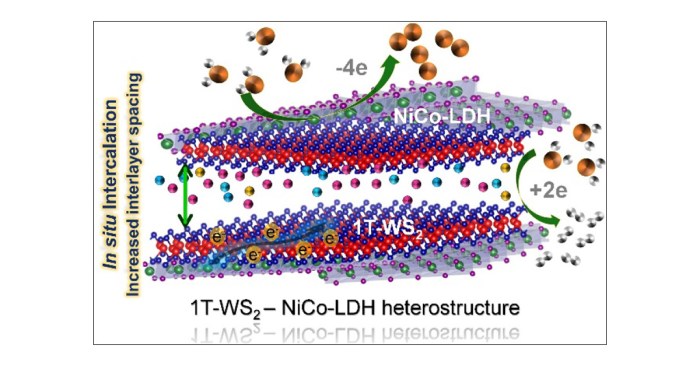
Applications of WOSSH
WOSSH is used in a variety of applications, including:
- As a catalyst for the oxidation of hydrocarbons
- As a photocatalyst for the production of hydrogen
- As a semiconductor in electronic devices
Advantages and Disadvantages, Tungsten oxygen oxygen oxygen sulphur hydrogen meaning
WOSSH has several advantages over other materials for these applications. It is a stable compound with a high melting point, making it ideal for use in high-temperature applications. It is also a good catalyst for the oxidation of hydrocarbons, making it a good choice for use in catalytic converters.
However, WOSSH also has some disadvantages. It is a black powder, which can make it difficult to use in some applications. It is also a relatively expensive material.
Case Studies
There are several case studies that demonstrate the successful use of WOSSH in a variety of applications. For example, WOSSH has been used as a catalyst for the oxidation of hydrocarbons in catalytic converters. It has also been used as a photocatalyst for the production of hydrogen.
In addition, WOSSH has been used as a semiconductor in electronic devices.
Frequently Asked Questions
What is the chemical formula for tungsten oxygen oxygen oxygen sulphur hydrogen?
The chemical formula for tungsten oxygen oxygen oxygen sulphur hydrogen is WOS2H.
What are the key properties of tungsten oxygen oxygen oxygen sulphur hydrogen?
Tungsten oxygen oxygen oxygen sulphur hydrogen exhibits high electrical conductivity, thermal stability, and resistance to corrosion.
Where is tungsten oxygen oxygen oxygen sulphur hydrogen commonly used?
Tungsten oxygen oxygen oxygen sulphur hydrogen finds applications in catalysis, energy storage, and electrochemical devices.