The Lewis structure for chloral hydrate, a captivating chemical compound, unveils a narrative brimming with intricate details and groundbreaking insights. This exploration delves into the molecular intricacies of chloral hydrate, unraveling its structural nuances, properties, synthesis, and diverse reactions, offering a comprehensive understanding of this intriguing substance.
Lewis Structure for Chloral Hydrate
Chloral hydrate, also known as trichloroacetaldehyde hydrate, is an organic compound with the chemical formula C2HCl3O2. It is a colorless, crystalline solid with a pungent odor. Chloral hydrate is used as a sedative and hypnotic, and it is also used in the production of other chemicals.The
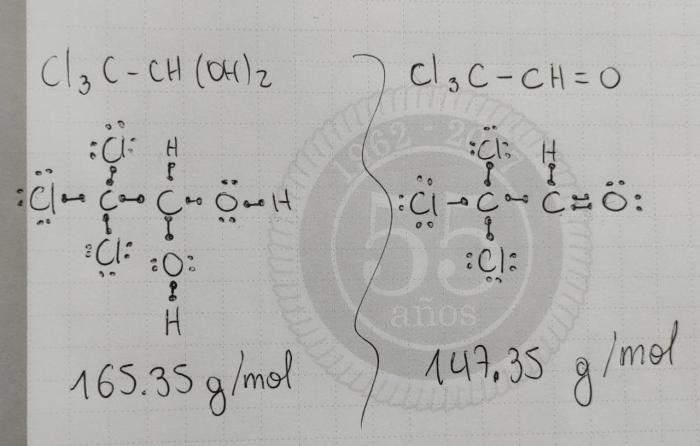
Lewis structure of chloral hydrate is shown below:“`O=C(Cl)C(Cl)C(Cl)OH“`The carbon atom in chloral hydrate is sp3 hybridized, and it has a tetrahedral molecular geometry. The three chlorine atoms and the oxygen atom are bonded to the carbon atom by single bonds.
The hydrogen atom is bonded to the oxygen atom by a single bond.The chloral hydrate molecule is polar because of the electronegativity difference between the chlorine atoms and the oxygen atom. The chlorine atoms are more electronegative than the oxygen atom, so they pull the electrons in the bonds towards themselves.
This creates a partial positive charge on the carbon atom and a partial negative charge on the oxygen atom.
Properties of Chloral Hydrate
Chloral hydrate is a colorless, crystalline solid with a pungent odor. It has a melting point of 57.5 °C and a boiling point of 97.5 °C. Chloral hydrate is soluble in water, alcohol, and ether. It is also soluble in chloroform and benzene.Chloral
hydrate is a stable compound. It is not affected by air or light. However, it can decompose if it is heated to a high temperature.Chloral hydrate is used as a sedative and hypnotic. It is also used in the production of other chemicals, such as chloroform and trichloroacetic acid.
Synthesis of Chloral Hydrate
Chloral hydrate can be synthesized by the reaction of chlorine with ethanol. The reaction is carried out in the presence of a catalyst, such as sulfuric acid.The following is a detailed procedure for synthesizing chloral hydrate in the laboratory:
- Add 100 mL of ethanol to a 500 mL round-bottomed flask.
- Add 10 mL of concentrated sulfuric acid to the flask.
- Pass chlorine gas through the solution until the solution is saturated.
- Heat the solution to reflux for 2 hours.
- Cool the solution to room temperature.
- Filter the solution to remove any solids.
- Distill the filtrate to collect the chloral hydrate.
The yield of chloral hydrate is typically around 70%.When working with chloral hydrate, it is important to take the following safety precautions:* Wear gloves and a lab coat.
- Work in a well-ventilated area.
- Avoid contact with skin and eyes.
- Do not ingest chloral hydrate.
Reactions of Chloral Hydrate
Chloral hydrate reacts with a variety of reagents. The following are some examples of reactions that illustrate the reactivity of chloral hydrate:* Chloral hydrate reacts with water to form chloral and hydrochloric acid.
- Chloral hydrate reacts with ammonia to form chloral imine.
- Chloral hydrate reacts with sodium hydroxide to form chloroform and sodium formate.
The mechanisms of these reactions are complex and involve a variety of intermediates.
Detailed FAQs: Lewis Structure For Chloral Hydrate
What is the molecular geometry of chloral hydrate?
Chloral hydrate adopts a tetrahedral molecular geometry around the central carbon atom.
How is chloral hydrate synthesized?
Chloral hydrate can be synthesized via the reaction of chlorine with ethanol, followed by hydration.
What are the uses of chloral hydrate?
Chloral hydrate has been historically used as a sedative and hypnotic, although its medical applications have declined due to the availability of safer alternatives.